
This can be easily obtained as the ratio is of the molecular weight of an element to the molecular weight of the compound. It is also possible to obtain the molecular formula from percent composition of an element for a given compound. The ratio when multiplied by 100 gives the percentage composition formula. %CE = (gE/gT) * 100 where CE is just a representation of the percentage composition of an element, gE is the total amount of element, gT is the total weight of the compound.

Mathematically to calculate the percentage by mass of an element in a compound the following formula is used: As can be seen from the above given formula, it is clear that the percentage composition of a compound can be expressed in terms of each of the individual elements present in the compound. Typically, the mass or weight of the elements and the compound is taken in grams. Percent Composition by Mass = (Mass of element / Molecular weight of compound) * 100 In simple terms, the formula for mass percentage of an element or the percent composition by mass is given as: The formula percentage composition or the formula for mass percentage is obtained as a ratio of the amount of each element present in the compound to the total sum of the individual elements of the compound, multiplied by 100 in order to obtain the percentage. Percent composition chemistry relies on the formula of percent composition by mass.

Properties of Percent Composition Formula This can be known by knowing the percentage composition of the element in a given compound which is obtained from the percent composition formula. Or in order to understand the contribution of a particular element in any of the stoichiometric calculations of a chemical compound of which the element is part of, it is necessary to know the amount of the given element in the compound.
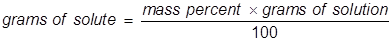
Sometimes in calculations about chemical reactions you need to identify the amount of an element in a given compound. You can also use our empirical formula calculator.Any chemical compound is composed of different constituent elements. The resulting molecular formula is C2H4O2. Next, Divide the molar mass of the substance by the molar mass of the empirical formula:įinally, multiply the number of atoms of each element in the empirical formula by this ratio. Find the molecular formula of a compound whose empirical formula is CH2O and molar mass is 60 g/mole.įirst, calculate the molar mass of the empirical formula:ġ(12 g/mole C) + 2(1 g/mole H) + 1(16 g/mole O) = 30 g/mole CH2O Once you have the empirical formula, you an find the molecular formula if the molar mass of the substance is known.
FORMULA FOR PERCENT BY MMASS HOW TO
How to find Molecular Formula from the Empirical Formula The resulting SIMPLEST WHOLE NUMBER empirical formula: C2H4O Find the empirical formula for this compound knowing that H = 1 g/mole, O = 16 g/mole and C = 12 g/mole.įirst, convert the grams of each element to moles by dividing by their molar mass: A compound is composed of 5.045g of carbon, 0.847g of hydrogen, and 3.36g of oxygen.

We can determine the empirical formula by using mass of each element in the compound data. How to find the Empirical Formula From Element Mass If one of the numbers was (say) 1.5, we have to multiply all the mole figures by 2. NOTE: In this case all mole figures represent whole numbers, so we can say that this is the SIMPLEST WHOLE NUMBER formula. The resulting SIMPLEST WHOLE NUMBER empirical formula: CH2O Then convert the grams of each element to moles by dividing by their molar mass:ĭivide each of the three mole figures by the lowest of the three in order to simplify the mole ratio. Find the empirical formula for this compound knowing that H = 1 g/mole, O = 16 g/mole and C = 12 g/mole. A compound is composed of 40% carbon, 6.67% hydrogen, and 53.3% oxygen. We can determine the empirical formula by using the proportion of each element in the compound data. How to calculate the Empirical Formula from Element Proportions
FORMULA FOR PERCENT BY MMASS SERIES
It is the simplest whole number non-reducible ratio formula for a molecular formula or compound.Ī molecular formula is a formula indicating the supposed molecular constitution of a compound, commonly consisting of a series of letters and numbers comprising the atomic symbols of each element present in a compound followed by the number of atoms of that element present in one molecule of the substance. An empirical formula is a chemical formula showing the ratio of elements in a compound rather than the total number of atoms.


 0 kommentar(er)
0 kommentar(er)
